Dagens Industri skriver i dagens tidning om Natural Cycles godkännande hos FDA.

Preventivmedelsappen Natural Cycles har fått godkänt av amerikanska läkemedelsverket och blir den först av sitt slag som får marknadsföras i USA.
Nu kan bolaget komma att ta in nytt kapital inför lanseringen.
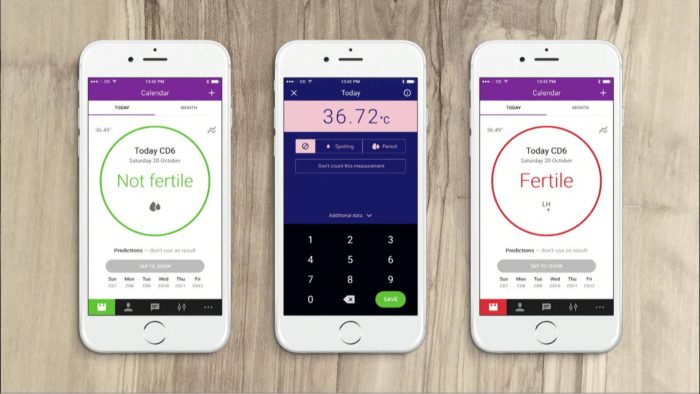
Appen, som fungerar genom att dagliga tester av kvinnans kroppstemperatur räknar ut vilka dagar där sex är säkert, har i ett test som myndigheten gjort inte fungerat i 1,8 procent av fallen när den används ”perfekt”. Testet, som gjort med 15.570 kvinnor, visade också att vid ”normalt” användande så blev 6,5 procent av de som använde appen gravid, då de inte använde alltid använde appen korrekt.
”Konsumenter använder i allt större utsträckning digital hälsoteknologi inför beslut som rör deras hälsa och den här nya appen kan ge en effektiv preventivmetod om den används försiktigt och korrekt. Men kvinnor bör vara medvetna om att ingen typ av preventivmedel fungerar perfekt, så en oplanerad graviditet kan bli resultatet även av korrekt användande av appen”, säger Terri Cornelison på FDA i ett pressmeddelande.
Natural Cycles har framgångsrikt marknadsförts i Sverige av olika storbloggare och en av stora finansiärer och delägare bakom Natural Cycles är Isabella Löwengrip .

FDA allows marketing of first direct-to-consumer app for contraceptive use to prevent pregnancy
The U.S. Food and Drug Administration today permitted marketing of the first mobile medical application (app) that can be used as a method of contraception to prevent pregnancy. The app, called Natural Cycles, contains an algorithm that calculates the days of the month a woman is likely to be fertile based on daily body temperature readings and menstrual cycle information, a method of contraception called fertility awareness. Designed for mobile devices, it is intended for use in pre-menopausal women aged 18 and older.
Natural Cycles requires women to take their temperature daily using a basal body thermometer, in the morning immediately upon waking, and to enter the reading into the app, which also tracks a user’s menstrual cycle. Basal body thermometers are more sensitive than regular thermometers and detect a minor rise in temperature, only about half of one degree Fahrenheit, around the time of ovulation. Women using the app for contraception should abstain from sex or use protection (such as a condom) when they see ”use protection” displayed on the app, which means they’re more likely to be fertile during those days.
”Consumers are increasingly using digital health technologies to inform their everyday health decisions, and this new app can provide an effective method of contraception if it’s used carefully and correctly,” said Terri Cornelison, M.D., Ph.D., assistant director for the health of women in the FDA’s Center for Devices and Radiological Health. ”But women should know that no form of contraception works perfectly, so an unplanned pregnancy could still result from correct usage of this device.”
Clinical studies to evaluate the effectiveness of Natural Cycles for use in contraception involved 15,570 women who used the app for an average of eight months. The app had a “perfect use” failure rate of 1.8 percent, which means 1.8 in 100 women who use the app for one year will become pregnant because they had sexual intercourse on a day when the app predicted they would not be fertile or because their contraceptive method failed when they had intercourse on a fertile day. The app had a ”typical use” failure rate of 6.5 percent, which accounted for women sometimes not using the app correctly by, for example, having unprotected intercourse on fertile days.
Natural Cycles should not be used by women who have a medical condition where pregnancy would be associated with a significant risk to the mother or the fetus or those currently using birth control or hormonal treatments that inhibit ovulation. Natural Cycles does not provide protection against sexually transmitted infections.
The FDA reviewed the Natural Cycles app through the de novo premarket review pathway, a regulatory pathway for novel, low-to-moderate-risk devices of a new type. Along with this authorization, the FDA is establishing criteria, called special controls, which clarify the agency’s expectations in assuring the accuracy, reliability and effectiveness in preventing pregnancy using apps indicated for contraception. These special controls, when met along with general controls, provide a reasonable assurance of safety and effectiveness for apps used for contraception. This action also creates a new regulatory classification, which means that subsequent devices with the same intended use may go through the FDA’s 510(k) process, whereby devices can obtain marketing authorization by demonstrating substantial equivalence to a predicate device.
Last year, the FDA released a Digital Health Innovation Action Plan to look at ways to provide clarity and find efficiency in how the agency regulates digital health technologies like the Natural Cycles app.
The FDA granted the marketing authorization for this app to Natural Cycles Nordic AB.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.